JINR researchers studied structural properties of Dsup, tardigrade radioprotective protein
News, 23 October 2024
DLNP and FLNP scientists at JINR, in collaboration with colleagues from Moscow Institute of Physics and Technology (Russia) and the University of South Florida (USF, USA), pioneered in studying the structural properties of the tardigrade radioprotective protein Dsup (Damage suppressor) and its complex with DNA. The study reveals new details of the highly effective DNA protection mechanism and extreme durability of one of the most radioresistant animals known to science, the tardigrade Ramazzottius varieornatus.
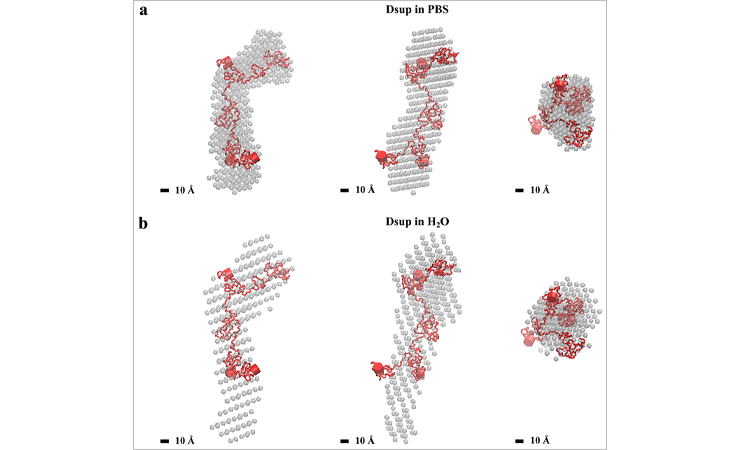 A model of the tardigrade protective protein Dsup in various solutions based on small-angle X-ray scattering data
A model of the tardigrade protective protein Dsup in various solutions based on small-angle X-ray scattering data
In order to determine the molecular mechanism contributing to the protection of DNA from radiation, the researchers conducted a structural and biological study of the tardigrade protein Dsup. For this purpose, they designed a protocol for the protein development and purification. Dsup was studied in a series of experiments using small angle X-ray scattering (SAXS), circular dichroism spectroscopy (CD), and bioinformatic analysis. It was found that Dsup is an intrinsically disordered protein, and the chosen methods are among the most suitable for the study of such flexible proteins with no rigid structure.
Disordered proteins have been actively studied in recent years as they play a significant part in a number of vital processes:
- DNA organisation in a cell;
- transcriptional regulation;
- the course of many diseases;
- survival in extreme conditions, and many others.
The most important result of the scientists’ work was the study of the intrinsic structure and molecular conformations of the Dsup protein under various physico-chemical conditions. In case of the extremely flexible Dsup protein, this made it possible to obtain averaged low-resolution atomic models and simulate a set of structures that more accurately describe the protein states in solution. Notably, the first analysis of the properties of the protein in interaction with DNA showed the formation of a fuzzy or structurally disordered Dsup-DNA complex, which indicates that the protein exists in this complex as a large number of diverse conformations. Based on the data obtained, the researchers assumed that the Dsup protein participates in the chromatin organization and that the disordered proteins act as protective molecules for DNA.
“Studying the extreme resistance mechanisms in living organisms requires understanding the processes at the molecular, genomic, and organismal levels. To do this, we plan to use new methods in the future, for example, cryo-electron microscopy,” a junior researcher at the DLNP JINR Sector of Molecular Genetics of the Cell Mikhail Zarubin said.
“The study of disordered proteins turned out to be a very difficult task. However, we managed to tackle it thanks to our hardworking and effective research team,” Head of the DLNP JINR Sector of Molecular Genetics of the Cell, Candidate of Biology Elena Kravchenko said.
The results of the first experimental study of the structural properties of the tardigrade Dsup protein were published in Scientific Reports in October 2024.