Ara-C radiosensitizer will help in proton therapy of melanoma
News, 23 May 2023
Employees of the Laboratory of Radiation Biology JINR have developed a fundamentally new method of increasing the biological efficiency of medical proton beams and gamma-therapeutic facilities. 1-β- D- arabinofuranosylcytosine (AraC), a well-known drug used in chemotherapy for leukaemia, in combination with proton radiation turned out to be able to inhibit the melanoma development in mice. Scientists are conducting further research that can lead to the use of this method in the treatment of oncological diseases.
One of the main methods of treatment for melanoma, especially for the early stages of the disease, is surgical excision of a tumour. However, in many cases, experts use other methods such as target immunopreparations, radiation therapy. Moreover, melanoma is one of the most radioresistant forms of malignant neoplasms. That is why scientists are looking for radiomodifiers capable of making a radiation therapy for this type of cancer more effective.
Scientific Leader of the Laboratory of Radiation Biology JINR RAS Corresponding Member Evgeny Krasavin spoke about the background of the research, “A new method continues the research started many years ago at the Laboratory. It is well known that irradiation of tumour cells damages their DNA that can be single-strand, potentially recoverable, and double-strand, leading to the cell death. Our specialists have shown that under the influence of 1-β-D-arabinofuranosylcytosine during the proton irradiation of cell cultures in vitro, the number of lethal DNA breaks in human cells increases.”
 LRB JINR Scientific Leader Evgeny Krasavin
LRB JINR Scientific Leader Evgeny Krasavin
In 2019, a group of LRB JINR described a fundamentally new application of a drug used in leukaemia chemotherapy, 1-β-D-arabinofuranosylcytosine (cytosine arabinoside, AraC; chemical name is 4-Amino-1-beta-D-arabinofuranosyl-2(1H)-pyrimidinone). Ara-C is incorporated in the end unit of a DNA molecule during repair, a restoration process of the initial DNA sequence, inhibiting it. The Ara-C inhibitor blocks the activity of DNA polymerases α and β involved in DNA synthesis during repair. Thus, it fixes single-strand DNA breaks. Moreover, if single-strand DNA tries to break in the presence of AraC, it leads to the formation of double-strand DNA breaks. Specialists have proved that AraC is a special radiosensitizer that transfer single-strand DNA breaks into double-strand ones when a cell tries to repair this damage. This feature distinguishes this drug from other DNA repair inhibitors. Based on the results of these studies, scientists received a JINR patent for the invention No. 2699670 authored by Evgeny Krasavin and others.
 Head of the Department of Radiation Biochemistry of Tsyb Medical Radiological Research Centre – a branch of NMIC of Radiology of the Ministry of Health of Russia, a leading researcher at the LRB JINR Department of Radiation Biology and Physiology Professor Irina Zamulaeva
Head of the Department of Radiation Biochemistry of Tsyb Medical Radiological Research Centre – a branch of NMIC of Radiology of the Ministry of Health of Russia, a leading researcher at the LRB JINR Department of Radiation Biology and Physiology Professor Irina Zamulaeva
It was extremely important to continue experiments on various types of normal and tumour cell cultures with experimental animals. Scientists conducted such studies in Obninsk under the guidance of Head of the Department of Radiation Biochemistry of Tsyb Medical Radiological Research Centre – a branch of NMIC of Radiology of the Ministry of Health of Russia, a leading researcher at the LRB JINR Department of Radiation Biology and Physiology, Professor Irina Zamulaeva. She explained that the action of Ara-C could be compared to a Trojan horse. Injected into the blood before irradiation, cytosine arabinoside is incorporated into the DNA of tumour cells. If there are single-strand DNA breaks during irradiation, their attempt to repair leads to their transformation into double-strand ones. It is important to note that single-strand DNA breaks take place ten times more often than double-strand ones during irradiation. Moreover, subsequent experiments have shown that the effect of Ara-C lasts more than one day, it is prolonged.
In 2021, specialists conducted first experiments on proton irradiation under the influence of Ara-C with animals (mice) inoculated with a melanoma tumour. Scientists divided 90 experimental animals inoculated with melanoma cells into a back paw into four groups: a control group without treatment, an “Ara-C” group that received only cytosine arabinoside chemotherapy, a “Protons” group with only irradiated animals, and an “Ara-C+Protons” group with animals treated by a combined method.
Scientists injected Ara-C at a concentration of 125 mg/kg into the tail vein of mice on day 12 after tumour cell transplantation. An hour after the administration of AraC, specialists once irradiated animals of the fourth group with a proton beam at a dose of 10 Gy. Scientists conducted the irradiation at Tsyb Medical Radiological Research Centre at the Prometheus Complex, which is the first Russian proton therapy complex certified for clinical use. The irradiation lasted for 4 minutes. In order to get exactly into the depth of the tumour, specialists used the so-called Bragg peak – a peak release of most of the energy of charged particles at the end of their travel. To reach the modified Bragg peak precisely in the tumour, scientists conducted irradiation through an 8 mm thick layer of polymethylmethacrylate. Every two-three days, starting from the moment of irradiation, they monitored the change in the size of tumour foci, calculating the average diameter and volume for each tumour.
The research has shown that the combination of Ara-C and proton radiation leads to the reduction of the number of tumour cells. In addition, a tumour becomes more sensitive to radiation. Moreover, the most important thing is that cancer stem cells (CSCs) lost their radioresistance. CSCs are a relatively small population of cells that forms new cells of a malignant neoplasm. They are responsible for maintaining its existence and proliferation. In many ways, CSCs are very similar to ordinary stem cells. For example, they can also evacuate various dyes and chemotherapy drugs from themselves. Therefore, they have better resistance to many antitumour drugs compared to the majority of non-stem cells. Specialists have been studying CSCs for already more than twenty years all over the world. Their inactivation is the most important task during treatment. Scientists have discovered that cytosine arabinoside effectively affects this type of cell as well. It opens up new prospects in the treatment of melanoma.
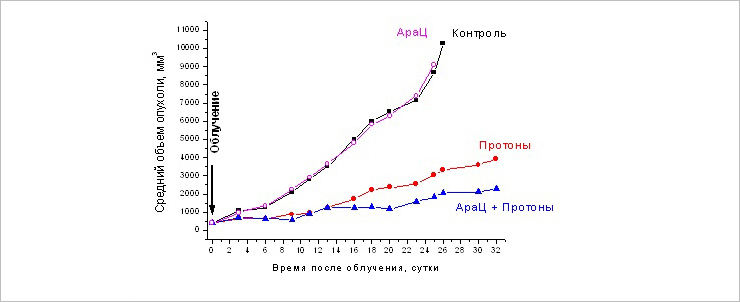 Change in the size of the primary melanoma focus B16 at different times after the single and combined action of AraC (125 mg / kg) and proton radiation at a dose of 10 Gy
Change in the size of the primary melanoma focus B16 at different times after the single and combined action of AraC (125 mg / kg) and proton radiation at a dose of 10 Gy
“Cancer stem cells have increased resistance to ionizing radiation and antitumor drugs. They can stay in tumour tissue after traditional treatment methods. Unfortunately, there is still no standard method to inactivate them and remove them from the tumour. However, if specialists use an inhibitor of DNA damage repair such as Ara-C, they lost their resistance. Therefore, they die in the same way as non-stem cells,” Irina Zamulaeva commented.
After combined exposure, the proportion of CSCs in tumours was reduced by 3.1 times compared to the one-time irradiation. Specialists have identified the ability of stem cells to evacuate a fluorescent lipophilic dye into the external environment and form the so-called lateral population of weakly coloured cells detected by flow cytometry.
Scientists detected the inhibition of tumour growth, especially starting from the ninth day after irradiation, in two groups, “Ara-C + Protons” and “Protons”, compared to the control one. Single administration of AraC had no effect on tumour growth. At the same time, starting from the 18th day, the combined method demonstrated significantly higher efficiency: the differences in the average volume of tumours between the groups reached 1.7 – 2.0 times. That means that the inhibition of tumour growth in the “Ara-C + Protons” group compared to the “Protons” one was 37-51%.
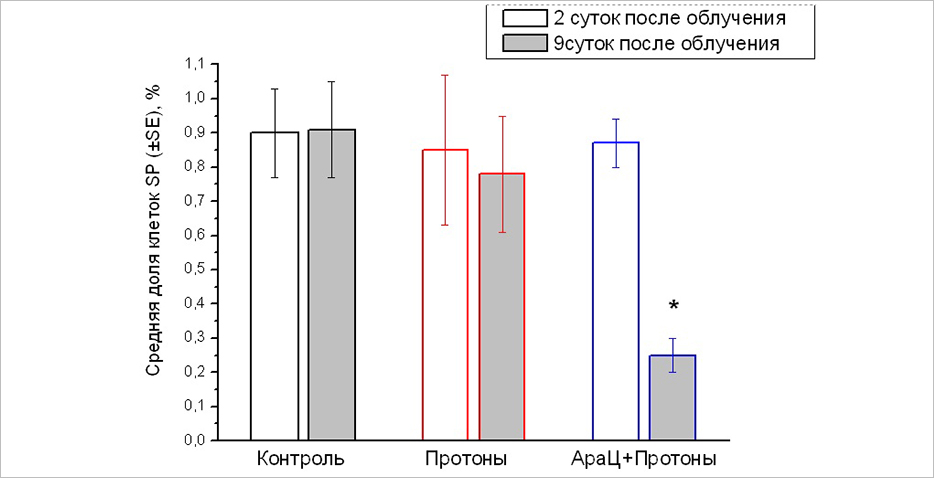 The proportion of CSCs in primary melanoma focus of line B16 in 2 days (white columns) and 9 days (gray columns) after proton irradiation and combined exposure to AraC and protons. *p=0.003 compared to the “Protons” group
The proportion of CSCs in primary melanoma focus of line B16 in 2 days (white columns) and 9 days (gray columns) after proton irradiation and combined exposure to AraC and protons. *p=0.003 compared to the “Protons” group
Molecular cell parameters of cell death and proliferative activity, i.e. processes, the balance of which determines radiation changes in the size of tumours, in the “AraC + Protons” and “Protons” groups approximately corresponded to each other. “Changes in the molecular cell parameters of cell death and proliferative activity are associated with inhibition of tumour growth after proton irradiation or combined exposure compared to the control group, but cannot explain the most pronounced effect of the inhibition of tumour growth in the “Ara-C + Protons” group. The only explanation of such an effect found during the research is associated with a decrease in the CSCs pool only after combined exposure,” an article “Radiobiological Effects of the Combined Action of 1-β-D-arabinofuranosylcytosine and Proton Radiation on B16 Melanoma In Vivo” published in issue No. 1, 2023, of the journal “Particles and Nuclei, Letters” based on the results of the research (authored by I. A. Zamulaeva, O. N. Matchuk, E. I. Selivanova, A. O. Yakimova, V. A. Mosina, S. N. Koryakin, A. D. Kaprin, A. V. Boreyko, A. N. Bugay, V. N. Chausov, E. A. Krasavin) explaines.
Scientists studied the effect of Ara-C in proton irradiation specifically for a reason. The dose distribution of such types of ionising radiation as gamma, X-ray, electron beam is not optimal for the treatment of tumours, unlike a beam of positively charged particles. Proton beams provide a conformal nature of the radiation of tumour tissue: maximum irradiation of the target – tumour, and minimal irradiation of surrounding healthy tissues, as Irina Zamulaeva explained.
Based on the results of the research, in June 2022, employees of Tsyb Medical Radiological Research Centre and LRB JINR received a patent for the invention No. 2774032 “Method for increasing the effectiveness of ionising radiation on melanoma “. “This patent describes single irradiation at a dose of 10 Gy. However, we are talking about several irradiation sessions during the treatment of patients, since it is impossible to give a large one-time dose to a patient. That is why, specialists carry out the so-called fractionated irradiation. The usual dose per fraction, per irradiation session, is about 2 Gy,” Irina Zamulayeva noted. A group of researchers is currently modelling the clinical situation, completing a course of fractionated irradiation in vivo.